Abstract
Background: Pyruvate kinase (PK) deficiency is a rare hereditary disease resulting in chronic hemolytic anemia. Iron overload is highly prevalent in patients (pts) with PK deficiency regardless of transfusion requirements, suggesting dyserythropoietic features. Iron overload can lead to serious complications including liver cirrhosis, cardiomyopathy, arrhythmia, sudden cardiac death, and endocrine dysfunction. In pts with PK deficiency, ineffective erythropoiesis combined with chronic hemolysis contributes to the manifestation of iron overload. Increased erythropoietic drive leading to erythroferrone-induced hepcidin suppression results in increased iron absorption and abnormal iron metabolism in PK deficiency. Furthermore, transfusions increase the existing burden of iron overload in PK deficiency. Mitapivat (AG-348) is a first-in-class, oral, allosteric activator of the red blood cell wildtype and mutant PK enzyme (PKR) that is approved by the US Food and Drug Administration for the treatment of hemolytic anemia in adults with PK deficiency, which has been shown to improve ineffective erythropoiesis and hemolysis. Previously reported data from ACTIVATE (NCT03548220) and its long-term extension (LTE) study (NCT03853798) also showed that treatment with mitapivat improved markers of iron homeostasis in adults with PK deficiency (up to 72 weeks [wks]). Here, we provide longer-term data from the LTE study on the impact of mitapivat on iron homeostasis and iron overload, and also assess liver iron concentration (LIC) changes in pts with iron overload at baseline (BL).
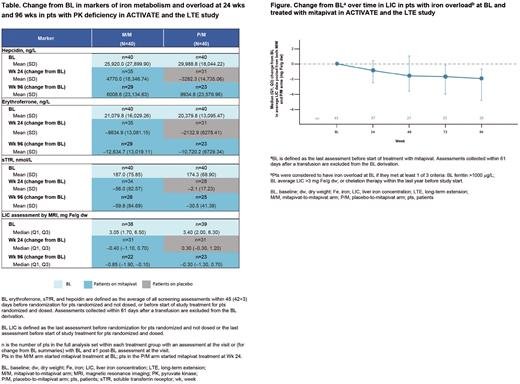
Methods: In ACTIVATE (double-blind, placebo [PBO]-controlled study), pts with PK deficiency who were not regularly transfused were randomized 1:1 to receive mitapivat or PBO. Pts who had demonstrated clinical benefit from mitapivat on completion of the fixed-dose period for ACTIVATE (24 wks), or who were assigned to the PBO arm in ACTIVATE, were eligible to continue in the LTE. All pts enrolled in the LTE received mitapivat. Pts from ACTIVATE who continued in the LTE were categorized into the mitapivat-to-mitapivat arm (M/M) or PBO-to-mitapivat arm (P/M). The ACTIVATE/LTE analysis assessed changes from BL over time (up to 96 wks), for both study arms, in markers related to iron homeostasis and overload, including erythroferrone, soluble transferrin receptor (sTfR), hepcidin, and LIC by magnetic resonance imaging. LIC changes over time in pts with evidence of iron overload at BL, were also assessed; pts were considered to have iron overload at BL if they met at least 1 of 3 criteria: BL ferritin >1000 mg/L, BL average LIC >3 mg Fe/g dry weight (dw), or chelation therapy within the last year before study start. For this calculation, BL was defined as the last assessment before start of treatment with mitapivat, and LIC data from pts in the M/M arm were pooled together with data from pts in the P/M arm and summarized over the time they were treated with mitapivat.
Results: 80 pts were included in the ACTIVATE/LTE analysis (M/M=40; P/M=40). Meaningful improvements in hepcidin, erythroferrone, sTfR, and LIC values were observed with mitapivat treatment previously; here, we confirm these meaningful improvements are continued up to 96 wks (Table). These measurements were improved early-on, within 24 wks of starting treatment in the M/M arm, but remained relatively unchanged in the P/M arm while on PBO. Similar improvements to the M/M arm were seen in the P/M arm after switching to mitapivat in the LTE. Among pts treated with mitapivat (N=78), 55.1% (n=43) met the criteria for iron overload at BL. These pts showed clinically meaningful and continued improvements in iron overload over time as measured by LIC (median [Q1, Q3] decrease from BL to Wk 96 of mitapivat treatment of -1.95 [-4.85, -0.70] mg Fe/g dw) (Figure).
Conclusions: Activation of PKR with mitapivat was associated with meaningful long-term improvements in iron homeostasis and overload. Mitapivat is the first disease-modifying pharmacotherapy shown to have beneficial effects on iron overload in pts with PK deficiency.
Disclosures
van Beers:Sobi: Research Funding; RR Mechatronics: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Global Blood Therapeutics: Consultancy; Sanofi: Consultancy. Al-Samkari:Forma: Consultancy; argenx: Consultancy; Rigel: Consultancy; Novartis: Consultancy; Amgen: Research Funding; Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Moderna: Consultancy; Dova: Consultancy, Research Funding. Grace:Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy; Novartis: Research Funding; Agios Pharmaceuticals: Consultancy, Research Funding. Barcellini:SOBI: Honoraria; Novartis: Honoraria; Momenta: Honoraria; Janssen: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Biocryst: Honoraria; Apellis: Honoraria; Alexion: Honoraria; Agios: Honoraria, Research Funding; Sanofi: Honoraria, Speakers Bureau; Bioverativ: Membership on an entity's Board of Directors or advisory committees. Glenthøj:Saniona: Research Funding; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Pharmacosmos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Dibacco:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Kosinski:Agios: Consultancy, Other: Shareholder. Xu:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company. Beynon:Agios: Current Employment, Other: Stockholder. Patel:Agios: Current Employment, Other: Stockholder. Porter:La Jolla Pharmaceuticals: Honoraria; Celgene: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; VIFOR: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Silence Therapeutics: Honoraria; Agios: Consultancy, Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Protagonism: Honoraria. Kuo:Alexion: Consultancy, Honoraria; Apellis: Consultancy; bluebird bio: Consultancy; Novartis: Consultancy, Honoraria; Celgene/BMS: Consultancy; Pfizer: Consultancy, Research Funding; Bioverativ/Sanofi/Sangamo: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal